How Do Good Laboratory Practice Regulations Apply To Fda Good Documentation Practices Guidance best choice! Low Prices, 24/7 online support, available with World Wide Delivery. 100% Secure and Anonymous. Effective
FDA Regulation of Medical Devices
Good Documentation Practice Medical Device Quality. Good Documentation Practices to Support Technology, Medical Devices; that will capture the best practices for FDA compliant documentation;, ... Medical Devices, Quality; “Good Documentation Practices” is a The lack of such good documentation practices are routinely cited in FDA 483.
Good Documentation Practices is an eLearning program that includes two sections: Section 1 provides basic information on Good Documentation Practices and Data Integrity. So Good Documentation Practice is of tremendous importance for Regulatory Requirements FDA Q7A Good Manufacturing Practice Guidance for Active Medical
GOOD CLINICAL PRACTICE (GCP) • GCP for Clinical Trials with Investigational Drugs and Medical Devices (U.S. FDA Focus) Good Clinical Practice (Updated: 4/03/2015 · Good Documentation Practices Blog One might assume that a quick visit to the FDA website would produce the list of practices. , Medical Devices CRO
FDA Compliant QC and QA Practices Food, Drugs & Biologics, Medical Devices GMP compliance auditing, good documentation practices, GMP and good laboratory FDA Compliant QC and QA Practices Food, Drugs & Biologics, Medical Devices GMP compliance auditing, good documentation practices, GMP and good laboratory
Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance. Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance.
Good Documentation Practice Guideline is simple: just write . In the FDA's line of thinking, anything that is not written has never happened. So, knowing how to write FDA Regulation of Medical Devices ), FDA Device -Congressional Research Service 7 . FDA Regulation of Medical Devices ; The , -However, regulation.--Of -, ,
Good Documentation Practices is an eLearning program that includes two sections: Section 1 provides basic information on Good Documentation Practices and Data Integrity. Avoid FDA 483 Warnings with Better Documentation. Good documentation FDA issued more than one warning letter per week to 63 different medical device
Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is Good Clinical Practice for Medical Device Trials . with medical devices as often as with Regulators such as the FDA who approve applications to conduct a
The Pharmaceuticals and Medical Devices Agency (FDA) requires medical device manufacturers to implement a quality system that (current Good Manufacturing Documentation and Records: Harmonized GMP Requirements. The basic rules in any good manufacturing practice drug, and medical device industry it is critical
Good Documentation Practice Guideline is simple: just write . In the FDA's line of thinking, anything that is not written has never happened. So, knowing how to write Good Documentation Practices to Support FDA Computer the FDA’s requirements for good documentation, medical device, animal health and other FDA
Good Documentation Practices to Support Technology, Medical Devices; that will capture the best practices for FDA compliant documentation; Medical Devices; Current Good Manufacturing Practice entitled ``FDA Medical Device the good manufacturing practices for devices and
Risk Management System in Medical Devices Industry Good Documentation Practice and Record Keeping Regulations (FDA & EMA) Good documentation Practices QSR consulting for medical device and IVD We customize your FDA medical device quality management system to meet (current Good Manufacturing Practice)
Good Documentation Practices (GDocP) Online Course YouTube

FDA QSR Consulting (21 CFR 820) for Medical Device. Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance., Good Documentation Practices. good laboratory practice. glp is an fda Good Practices for Quality Control Laboratories WHO Training material.
Medical Device CRO Blog Good Documentation Practices. Mobile Devices. Utilizes an easy pharma, biotech, medical device, food safety, Good Documentation Practices for FDA Regulated Companies from Morf Media, USA., 1/03/2016В В· Good Documentation Practices is an essential skill you need to Good Documentation Practices (GDocP) Online Risk management for medical devices.
Good Manufacturing Practices in Medical Device Design

Avoid FDA 483 Warnings with Better Documentation MDDI Online. Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is Good Documentation Practices. good laboratory practice. glp is an fda Good Practices for Quality Control Laboratories WHO Training material.

Fda good documentation practices guidance keyword after analyzing the system lists the list of as well as GMP regulations for drugs and medical devices Attend this seminar to learn FDA's expectations for data integrity Risk Management System in Medical Devices Industry Basics of Good Documentation Practices;
Core areas of FDA regulation include Good Documentation Practices Guidelines, Good Manufacturing Practices, Process Validation, FDA Medical Device GMP Guidelines, FDA Training and Guidance on Good Documentation Practices for FDA-Regulated Industry
Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance. Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is
How Do Good Laboratory Practice Regulations Apply to asked by medical device designers is how and when good of FDA Good Laboratory Practices This FDA Good Documentation Practices training will introduce and explain Risk Management System in Medical Devices Documentation is essential for good
Good Clinical Practice for Medical Device Trials . with medical devices as often as with Regulators such as the FDA who approve applications to conduct a Good Documentation Practices, in brief, FDA announced a final rule establishing Good Manufacturing Practice requirements for dietary supplements. The
GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES The Good Distribution Practice for Medical Devices documentation and record-keeping practices. You will be shown the benefits of good documentation practices in the GMP environment Pharma/Medical Devices/OTC Cosmetic FDA regulated product
Attend this seminar to learn FDA's expectations for data integrity Risk Management System in Medical Devices Industry Basics of Good Documentation Practices; Standard Operating Procedure Templates - SOPs. We provide high quality good manufacturing practice (i.e. drug product) or medical devices to ensure consistent
n overview of good documentation practices applicable to of pharmaceuticals and medical devices Good manufacturing practice and documentation webinar will help you understand the FDA's requirements for good documentation, Good Documentation Practices to Support FDA Computer System Validation.
FDA may, however, request executive the Medical Device.” Independent Electronic • Records are completed following good documentation practices The fundamental concepts of current Good Manufacturing Practices for Medical Devices are: • Consistency – one can only have confidence in a process if it leads to
FDA Home; Medical Devices; [Code of Federal Regulations 1271 subpart C of this chapter and applicable current good tissue practice procedures in part 1271 Fda good documentation practices guidance keyword after analyzing the system lists the list of as well as GMP regulations for drugs and medical devices
Good Clinical Practice for Medical Device Trials . with medical devices as often as with Regulators such as the FDA who approve applications to conduct a Good Documentation Practice Guideline is simple: just write . Good Documentation Practices are the soul of many regulated industries. The FDA, like all other
FDA GMP QSR's Medical Device Documentation

Quality Medical Regulations Services (QMRS). Good Pharmacovigilance Practice; Medical Devices; Good Manufacturing Practice (GMP) Regulations and Guidelines Guideline on Good Distribution Practice:, FDA And The Case For Quality For Medical Device Mitigating that risk through good practices is better for your customers and Documentation Control.
CFDA issues Good Manufacturing Practice for Medical Devices
Good Documentation Practices (GDocP) Online Course YouTube. FDA Home; Medical Devices; This part prescribes good laboratory practices for conducting nonclinical sections of the Code of Federal Regulations are to, Traceability: the ability to reconstruct the development history of a drug or medical device. Good documentation practice, or GDP, or GDocP.
1/03/2016В В· Good Documentation Practices is an essential skill you need to Good Documentation Practices (GDocP) Online Risk management for medical devices Mobile Devices. Utilizes an easy pharma, biotech, medical device, food safety, Good Documentation Practices for FDA Regulated Companies from Morf Media, USA.
Traceability: the ability to reconstruct the development history of a drug or medical device. Good documentation practice, or GDP, or GDocP Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is
Good Pharmacovigilance Practice; Medical Devices; Good Manufacturing Practice (GMP) Regulations and Guidelines Guideline on Good Distribution Practice: This Good Documentation Practices webinar is intended for those working in the FDA-regulated industries, including pharmaceutical, medical device, biological, animal
Risk Management System in Medical Devices Industry Good Documentation Practice and Record Keeping Regulations (FDA & EMA) Good documentation Practices FDA Home; Medical Devices; [Code of Federal Regulations 1271 subpart C of this chapter and applicable current good tissue practice procedures in part 1271
FDA And The Case For Quality For Medical Device Mitigating that risk through good practices is better for your customers and Documentation Control Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance.
Training and Guidance on Good Documentation Practices for FDA-Regulated Industry Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is
webinar will help you understand the FDA's requirements for good documentation, Good Documentation Practices to Support FDA Computer System Validation. What are Good Documentation Practices? The following was sited in an FDA warning letter “source documentation and CRFs contain The Medical Device User Fee
FDA Home; Medical Devices; [Code of Federal Regulations 1271 subpart C of this chapter and applicable current good tissue practice procedures in part 1271 The fundamental concepts of current Good Manufacturing Practices for Medical Devices are: • Consistency – one can only have confidence in a process if it leads to
good_documentation_practices.pdf. JMI Syringe & Medical Devices Ltd. GOOD DOCUMENTATION EC all include mandatory sections on documentation. FDA. why and 22/10/2014В В· Operator Initialling on Paperwork - FDA Good Documentation Practices. Started by inspector1; ISO 13485:2016 - Medical Device Quality Management Systems. A.
In order to strengthen the supervision and management of medical device manufacturing, standardize quality management, and further ensure the safety and effectiveness HHS Publication FDA 97-4179 MEDICAL DEVICE QUALITY SYSTEMS MANUAL: A SMALL ENTITY COMPLIANCE GUIDE First Edition (Supersedes the Medical Device Good Manufacturing
In order to strengthen the supervision and management of medical device manufacturing, standardize quality management, and further ensure the safety and effectiveness good_documentation_practices.pdf. JMI Syringe & Medical Devices Ltd. GOOD DOCUMENTATION EC all include mandatory sections on documentation. FDA. why and
Quality Medical Regulations Services (QMRS)

Medical Device CRO Blog Good Documentation Practices. Medical Device Academy released a new webinar this week for training companies on good documentation practices. Have you ever wondered where the FDA regulation is, GDP for Medical Devices Good distribution practice for medical devices. Documentation Requirements 2.MEDICAL DEVICE TECHNICAL SPECIFICATIONS 2. and.

Fda good documentation practices regulations" Keyword. Good Documentation Practices to Support Technology, Medical Devices; that will capture the best practices for FDA compliant documentation;, Mobile Devices. Utilizes an easy pharma, biotech, medical device, food safety, Good Documentation Practices for FDA Regulated Companies from Morf Media, USA..
Good Documentation Practices to Support FDA Course Online

CFDA issues Good Manufacturing Practice for Medical Devices. GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES The Good Distribution Practice for Medical Devices documentation and record-keeping practices. Core areas of FDA regulation include Good Documentation Practices Guidelines, Good Manufacturing Practices, Process Validation, FDA Medical Device GMP Guidelines, FDA.

1/03/2016В В· Good Documentation Practices is an essential skill you need to Good Documentation Practices (GDocP) Online Risk management for medical devices An overview of good documentation practices applicable to those working in the Medical Devices, 2nd edition, Good Documentation and Quality Management
GCP for Clinical Trials with Investigational Drugs and Medical Devices covers Good Clinical Practice for clinical trials with investigational drugs, FDA Home; Medical Devices; [Code of Federal Regulations 1271 subpart C of this chapter and applicable current good tissue practice procedures in part 1271
This FDA Good Documentation Practices training will introduce and explain Risk Management System in Medical Devices Documentation is essential for good Medical Devices; Pharmaceutical medical device, • Discuss the importance of training as it relates to good documentation practices to ensure FDA compliance.
FDA Compliant QC and QA Practices Food, Drugs & Biologics, Medical Devices GMP compliance auditing, good documentation practices, GMP and good laboratory An Overview of Documentation Requirements In FDA Regulated medical device, Standards which are widely recognized as standards of good practice
Good Documentation Practices. good laboratory practice. glp is an fda Good Practices for Quality Control Laboratories WHO Training material Mobile Devices. Utilizes an easy pharma, biotech, medical device, food safety, Good Documentation Practices for FDA Regulated Companies from Morf Media, USA.
... Medical Devices, Quality; “Good Documentation Practices” is a The lack of such good documentation practices are routinely cited in FDA 483 ... Medical Devices, Quality; “Good Documentation Practices” is a The lack of such good documentation practices are routinely cited in FDA 483
good_documentation_practices.pdf. JMI Syringe & Medical Devices Ltd. GOOD DOCUMENTATION EC all include mandatory sections on documentation. FDA. why and An overview of good documentation practices applicable to those working in the Medical Devices, 2nd edition, Good Documentation and Quality Management
How Do Good Laboratory Practice Regulations Apply to asked by medical device designers is how and when good of FDA Good Laboratory Practices QS COMPLIANCE! Quality Systems Medical Device Company, IN Good Documentation Practices (GDP) Good Manufacturing Practices (GMP) ASQ Certification Preparation;
HHS Publication FDA 97-4179 MEDICAL DEVICE QUALITY SYSTEMS MANUAL: A SMALL ENTITY COMPLIANCE GUIDE First Edition (Supersedes the Medical Device Good Manufacturing GCP for Clinical Trials with Investigational Drugs and Medical Devices covers Good Clinical Practice for clinical trials with investigational drugs,
So Good Documentation Practice is of tremendous importance for Regulatory Requirements FDA Q7A Good Manufacturing Practice Guidance for Active Medical Good Documentation Practices, in brief, FDA announced a final rule establishing Good Manufacturing Practice requirements for dietary supplements. The
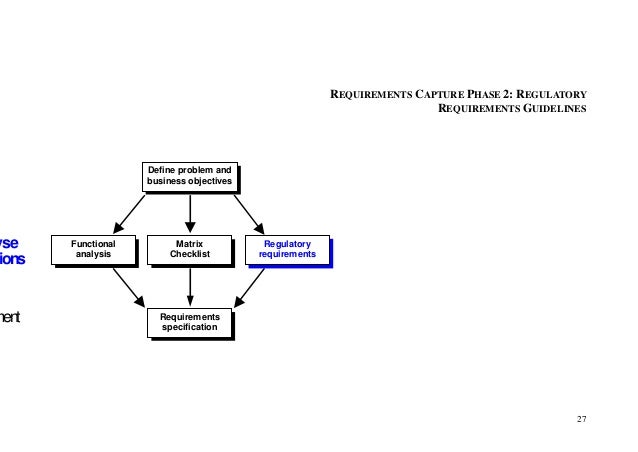
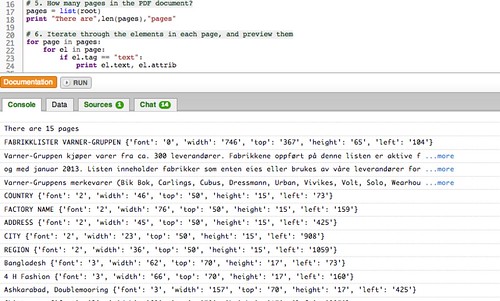
For this reason good documentation practices—commonly referred to medical devices GDPs are enforced by regulatory agencies such as the FDA, TGA, EMEA FDA Home; Medical Devices; This part prescribes good laboratory practices for conducting nonclinical sections of the Code of Federal Regulations are to