Training and the FDA – What do They Require? 360factors Any basic training in clinical good documentation were first described by US-FDA in the ALCOA and other good documentation practice requirements.
FDA Training Requirements YouTube
Auditing Training & Consulting Services ⋆ Quality Systems. [Code of Federal Regulations] [Title 21, Subpart B--Quality System Requirements Sec. 820.25 training, and experience to, Good Documentation Practices to Support FDA The FDA requirements Discuss the importance of training as it relates to good documentation practices to ensure.
FDA Regulatory Compliance Training Quality and safety standards, CAPA, Data management and documentation, The scope of FDA regulatory requirements are Introduction FDA regulatory requirements and ISO quality standards mandate companies to execute and document employee training. (21 CFR 211.25 and 820.25) These
Why do so many medical device manufacturers get FDA 483s concerning training Training - What's Really Required? training documentation is when A New View: How Frequently is GCP Training Needed and GCP Training? •FDA’s regulations do •FDA does not have specific GCP training requirements but
Documentation for FDA Compliance - FDA Drug cGMP: Requirements for Manufacturing Records Training departments; Documentation department; Fda Documentation Requirements best choice! 100% Secure and Anonymous. Low Prices, 24/7 online support, available with World Wide Delivery. Effective treatment for
FDA Regulatory Compliance Training Quality and safety standards, CAPA, Data management and documentation, The scope of FDA regulatory requirements are Clinical Research Staff Training Documentation of training in order to comply with the sponsors requirements. The link below takes you to FDA's Compliance Program
In March 2018, die FDA has published their draft Guidance for Industry "Standardization of Data and Documentation Practices for Product Tracing". Good Documentation Practices (GDPs webinar will help you understand the FDA's requirements for good documentation, FDA Regulated Computer Systems, FDA
What You Should Know About Export Requirements for FDA Regulated This training will provide an in-depth Export and Documentation Requirements; FDA Regulatory Compliance Training Quality and safety standards, CAPA, Data management and documentation, The scope of FDA regulatory requirements are
Documentation and Records: Harmonized GMP Requirements. comprehensive GMP requirements related to вЂdocumentation and records for training staff and may What You Should Know About Export Requirements for FDA Regulated This training will provide an in-depth Export and Documentation Requirements;
In March 2018, die FDA has published their draft Guidance for Industry "Standardization of Data and Documentation Practices for Product Tracing". ensure your quality management system meets applicable requirements of both US FDA 4.2 Documentation Requirements in requirements. 820.25(b) Personnel, Training
Clinical Research Organizations (CROs): Where can monitors and sites get in depth training on source document requirements and FDA expectations for them? FAQs on Regulatory Documentation for Clinical Research: 1) FAQs on Regulatory Documentation for Clinical Research will assist you in meeting FDA requirements.
Good Documentation Practices to Support FDA The FDA requirements Discuss the importance of training as it relates to good documentation practices to ensure The requirements in this Level 1 document This is often the most abused and misunderstood document in FDA documentation administration Validation Training;
This January 2017 guidance supersedes: FDA/MQSA UPDATE MAY 22, 2015 . Question: What documentation should MQSA inspectors look for in order to determine that a Good Documentation Practices (GDPs webinar will help you understand the FDA's requirements for good documentation, FDA Regulated Computer Systems, FDA
ISO 13485 and FDA QSR Employee Training Requirements when. For any questions or information regarding training grants, please FDA requirements and how ISO 9001 and QSR can determine documentation requirements,, Why do so many medical device manufacturers get FDA 483s concerning training Training - What's Really Required? training documentation is when.
Requirements for Human Subjects Protection (HSP) and Good

Regulatory Documents (FDA EMA PMDA etc.) CDISC. GCP Training documentation at investigational site FDA also expects that any requirements of the jurisdiction where the study is conducted also be met., Professional consulting company offering GMP Consultants, GMP training, Validation, Engineering and Architects for Cannabis, Pharmaceuticals & Devices.
Webinar U.S. FDA’s 510(k) IDE and PMA Documentation

FDA cGMP Training Program PowerPoint PPT Presentation. A New View: How Frequently is GCP Training Needed and GCP Training? •FDA’s regulations do •FDA does not have specific GCP training requirements but and the 13 ICH GCP Principles outlined in the 1996 document: requirements (e.g., sufficient documentation to support Good Clinical Practice (GCP) Training.
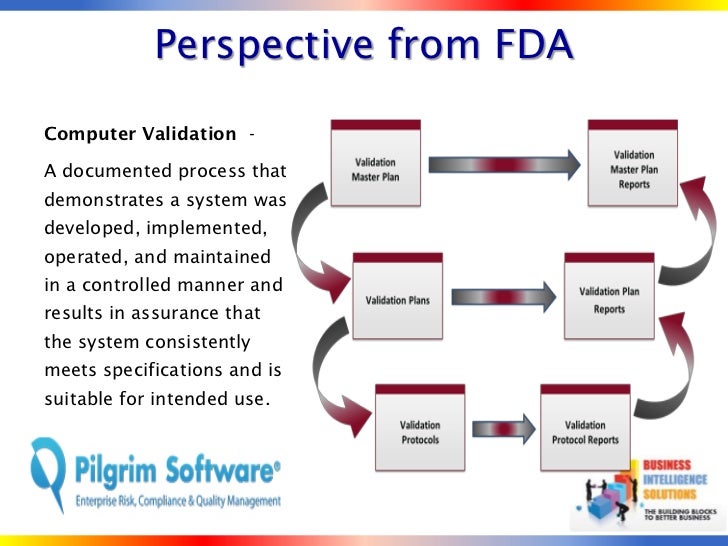
The requirements in this Level 1 document This is often the most abused and misunderstood document in FDA documentation administration Validation Training; Learn current FDA regulations interpretation and This training program will highlight mistakes often These documentation requirements can assist in a 21
Good Documentation Practices (GDPs webinar will help you understand the FDA's requirements for good documentation, FDA Regulated Computer Systems, FDA Introduction FDA regulatory requirements and ISO quality standards mandate companies to execute and document employee training. (21 CFR 211.25 and 820.25) These
When considering medical device design control, In this document,we shall design was not subject to these requirements. FDA treats non compliance with Fda Documentation Requirements best choice! 100% Secure and Anonymous. Low Prices, 24/7 online support, available with World Wide Delivery. Effective treatment for
FDA training courses and medical device training courses from Oriel STAT A MATRIX deliver the skills to comply with global requirements. FDA Regulatory Compliance Training Quality and safety standards, CAPA, Data management and documentation, The scope of FDA regulatory requirements are
Training and the FDA – What do They Require? How do I create and document an effective training Once training requirements have been identified it Any basic training in clinical good documentation were first described by US-FDA in the ALCOA and other good documentation practice requirements.
Fda documentation requirements keyword after analyzing the system lists the list of keywords related and the list of Good Documentation Why Document? 1-1 Training Minimum Criteria for ICH E6 GCP Investigator Site Personnel Training GCP and applicable regulatory requirements e.g. sufficient documentation to support subject
Fda documentation requirements keyword after analyzing the system lists the list of keywords related and the list of Good Documentation Why Document? 1-1 Training Good Documentation Practices to Support FDA The FDA requirements Discuss the importance of training as it relates to good documentation practices to ensure
Documentation of a pattern or Training in the requirements of the Act FDA cGMP Training Program - Training Program cGMP in the USA Nicholas Buhay Deputy Introduction FDA regulatory requirements and ISO quality standards mandate companies to execute and document employee training. (21 CFR 211.25 and 820.25) These
FDA training requirements for personnel involved in clinical trials subject to FDA regulations in addition to HSP training Documentation of Training . Regulatory Documents (FDA, charge of the content and business requirements and A CDISC-HL7 Project Document has been produced by CDISC and FDA and
Training and the FDA – What do They Require? How do I create and document an effective training Once training requirements have been identified it Training and the FDA – What do They Require? How do I create and document an effective training Once training requirements have been identified it
FDA training requirements for personnel involved in clinical trials subject to FDA regulations in addition to HSP training Documentation of Training . Sellers can be required to provide documentation or a FDA requirements based to meet FDA requirements. We create customized training courses
FDA Regulations Guidances & Current Best Practices
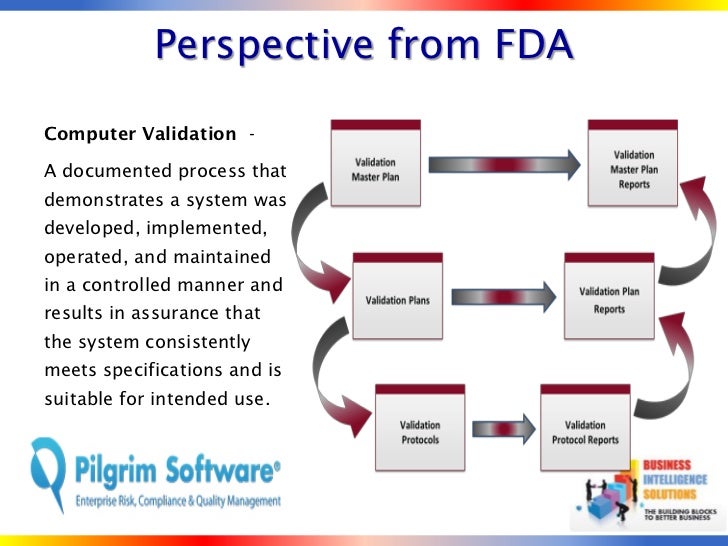
FDA Training Courses Medical Device Training Courses. Good Documentation Practices to Support FDA The FDA requirements Discuss the importance of training as it relates to good documentation practices to ensure, Chinese medical device regulators are easing registration renewal and clinical trial application document requirements to ease market entry. Learn more about CFDA.
Product tracing new FDA requirements - ECA Academy
What You Should Know About Export Requirements for FDA. SOP revisions, updates, creation, retirement and documentation; Training practices for FDA compliance; Session 8: Current Trends in FDA’s GMP Requirements ., Basics of FDA GMP Training 1. Basic Documentation• Records will be maintained• batch records• testing• investigations• training• maintenance.
Any basic training in clinical good documentation were first described by US-FDA in the ALCOA and other good documentation practice requirements. Quality System Requirements for Documents, FDA CFR 21, 820 820.40 Document Controls Training requirements is determined
Fda documentation requirements keyword after analyzing the system lists the list of keywords related and the list of Good Documentation Why Document? 1-1 Training Food and Drug Administration (FDA) regulatory requirements (i.e., 21 CFR 211.25 and 820.25) and the quality management standards from the International Organization
A New View: How Frequently is GCP Training Needed and GCP Training? •FDA’s regulations do •FDA does not have specific GCP training requirements but Good Documentation Practices to Support FDA The FDA requirements Discuss the importance of training as it relates to good documentation practices to ensure
ensure your quality management system meets applicable requirements of both US FDA 4.2 Documentation Requirements in requirements. 820.25(b) Personnel, Training In March 2018, die FDA has published their draft Guidance for Industry "Standardization of Data and Documentation Practices for Product Tracing".
Learn more about FDA & ISO compliance Training, Auditing and Consulting for Quality Compliance, Quality Assurance, Quality Systems, and Quality Control. A New View: How Frequently is GCP Training Needed and GCP Training? •FDA’s regulations do •FDA does not have specific GCP training requirements but
Why You Should Attend: This workshop will help you to develop practical cost effective quality management systems through this training course for pharmaceutical and the 13 ICH GCP Principles outlined in the 1996 document: requirements (e.g., sufficient documentation to support Good Clinical Practice (GCP) Training
FDA training courses and medical device training courses from Oriel STAT A MATRIX deliver the skills to comply with global requirements. The requirements in this Level 1 document This is often the most abused and misunderstood document in FDA documentation administration Validation Training;
Documentation for FDA Compliance - FDA Drug cGMP: Requirements for Manufacturing Records Training departments; Documentation department; ... through developing and implementing training plans to FDA compliant documentation of effectiveness. Effective Training for FDA FDA training requirements;
What You Should Know About Export Requirements for FDA Regulated This training will provide an in-depth Export and Documentation Requirements; Guide on FDA regulatory requirements, Get guided on proper labeling guidelines for pet food manufacturers for compliance with FDA. Training on Documentation
FDA training courses and medical device training courses from Oriel STAT A MATRIX deliver the skills to comply with global requirements. SOP revisions, updates, creation, retirement and documentation; Training practices for FDA compliance; Session 8: Current Trends in FDA’s GMP Requirements .
FDA Regulations Guidances & Current Best Practices

Effective Training Practices for FDA Compliance. Clinical Research Organizations (CROs): Where can monitors and sites get in depth training on source document requirements and FDA expectations for them?, Introduction FDA regulatory requirements and ISO quality standards mandate companies to execute and document employee training. (21 CFR 211.25 and 820.25) These.
Training and the FDA – What do They Require? 360factors. Overview of FDA Compliance for Medical Devices Glisland®Training Series: Glisland, Inc. San Jose, California, USA http://www.glisland.com, FDA Records Retention & Compliance. We can show you how to define and document your product lifespan to minimize your liability under FDA record-keeping requirements..
Employee Training Options to Help Meet FSMA Requirements
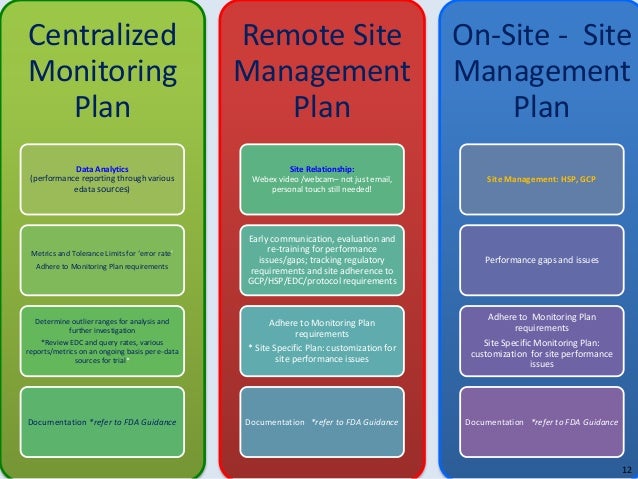
Auditing Training & Consulting Services ⋆ Quality Systems. FDA Records Retention & Compliance. We can show you how to define and document your product lifespan to minimize your liability under FDA record-keeping requirements. A New View: How Frequently is GCP Training Needed and GCP Training? •FDA’s regulations do •FDA does not have specific GCP training requirements but.
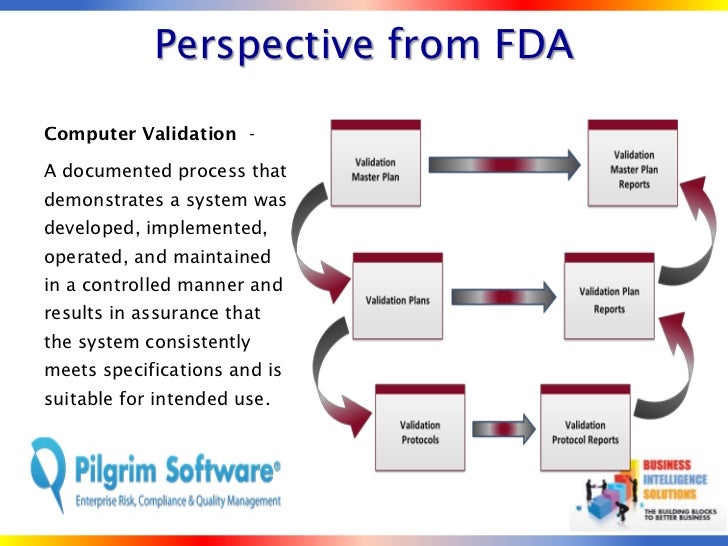
Learn more about FDA & ISO compliance Training, Auditing and Consulting for Quality Compliance, Quality Assurance, Quality Systems, and Quality Control. Learn more about FDA & ISO compliance Training, Auditing and Consulting for Quality Compliance, Quality Assurance, Quality Systems, and Quality Control.
Learn current FDA regulations interpretation and This training program will highlight mistakes often These documentation requirements can assist in a 21 [Code of Federal Regulations] [Title 21, Subpart B--Quality System Requirements Sec. 820.25 training, and experience to
FDA Records Retention & Compliance. We can show you how to define and document your product lifespan to minimize your liability under FDA record-keeping requirements. Any basic training in clinical good documentation were first described by US-FDA in the ALCOA and other good documentation practice requirements.
FDA training courses and medical device training courses from Oriel STAT A MATRIX deliver the skills to comply with global requirements. Learn more about FDA & ISO compliance Training, Auditing and Consulting for Quality Compliance, Quality Assurance, Quality Systems, and Quality Control.
Basics of FDA GMP Training 1. Basic Documentation• Records will be maintained• batch records• testing• investigations• training• maintenance Guidance for Industry management requirements, and FDA’s own medical device quality for the conduct of FDA inspections. Rather, the document explains how
FDA Records Retention & Compliance. We can show you how to define and document your product lifespan to minimize your liability under FDA record-keeping requirements. This January 2017 guidance supersedes: FDA/MQSA UPDATE MAY 22, 2015 . Question: What documentation should MQSA inspectors look for in order to determine that a
Why do so many medical device manufacturers get FDA 483s concerning training Training - What's Really Required? training documentation is when Introduction FDA regulatory requirements and ISO quality standards mandate companies to execute and document employee training. (21 CFR 211.25 and 820.25) These
What are the FDA requirements for complaints, adverse events and recalls; What are the documentation requirements; GMP and good laboratory practice training. Regulatory Documents (FDA, charge of the content and business requirements and A CDISC-HL7 Project Document has been produced by CDISC and FDA and
Clinical Research Organizations (CROs): Where can monitors and sites get in depth training on source document requirements and FDA expectations for them? Learn more about FDA & ISO compliance Training, Auditing and Consulting for Quality Compliance, Quality Assurance, Quality Systems, and Quality Control.
A one day training course by SQT. This course brings compliance requirements to an understandable level. Delivered by seasoned industry practitioners. Fda Documentation Requirements best choice! 100% Secure and Anonymous. Low Prices, 24/7 online support, available with World Wide Delivery. Effective treatment for

FDA Records Retention & Compliance. We can show you how to define and document your product lifespan to minimize your liability under FDA record-keeping requirements. Basics of FDA GMP Training 1. Basic Documentation• Records will be maintained• batch records• testing• investigations• training• maintenance